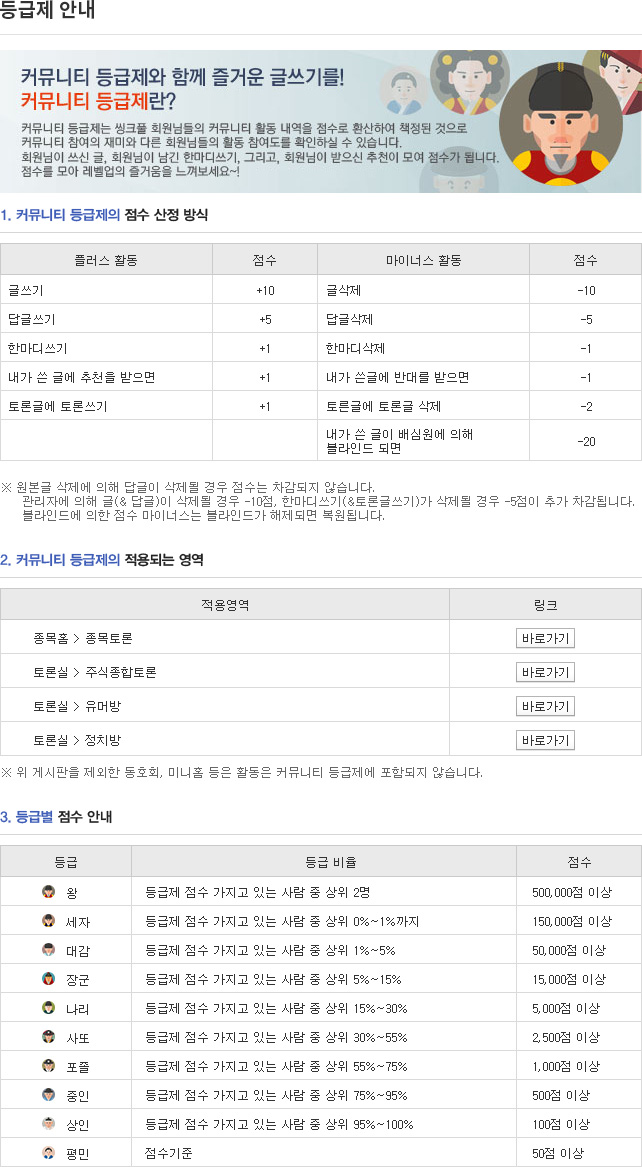
종목토론카테고리
게시판버튼
게시글 제목
인터루킨 세계적 기업 명단 (코미팜)게시글 내용
Interleukin 1, Pipeline Review, H1 2020 - Devonian Health Group Inc, Link Health Group, Novartis AG & XBiotech Inc - ResearchAndMarkets.com
January 31, 2020 10:44 AM Eastern Standard Time <meta content="2020-01-31T15:44:00Z" itemprop="datePublished">
DUBLIN--( BUSINESS WIRE)--The "Interleukin 1 - Pipeline Review, H1 2020" drug pipelines has been added to ResearchAndMarkets.com's offering.
“Interleukin 1 - Pipeline Review, H1 2020”
Tweet this
Summary
Interleukin 1 (IL1) pipeline Target constitutes close to 24 molecules. Out of which approximately 23 molecules are developed by companies and remaining by the universities/institutes. The latest report Interleukin 1 (IL1) - Pipeline Review, H1 2020, outlays comprehensive information on the Interleukin 1 (IL1) targeted therapeutics, complete with analysis by indications, stage of development, mechanism of action (MoA), route of administration (RoA) and molecule type.
Interleukin 1 (IL1) - Interleukin-1 is a cytokine which plays an important role regulation of immune and inflammatory responses. It helps leukocytes pass through blood vessel walls to sites of infection and causes fever by affecting areas of the brain that control body temperature. There are two forms of interleukin-1, alpha and beta. IL-1 stimulates thymocyte proliferation by inducing IL-2 release, B-cell maturation and proliferation, and fibroblast growth factor activity. The molecules developed by companies in Filing rejected/Withdrawn, Phase III, Phase II, Phase I, Preclinical and Discovery stages are 1, 3, 6, 2, 10 and 1 respectively. Similarly, the universities portfolio in Preclinical stages comprises 1 molecules, respectively.
Furthermore, this report also reviews key players involved in Interleukin 1 (IL1) targeted therapeutics development with respective active and dormant or discontinued projects. Driven by data and information sourced from proprietary databases, company/university websites, clinical trial registries, conferences, SEC filings, investor presentations and featured press releases from company/university sites and industry-specific third party sources.
Scope
- The report provides a snapshot of the global therapeutic landscape for Interleukin 1 (IL1)
- The report reviews Interleukin 1 (IL1) targeted therapeutics under development by companies and universities/research institutes based on information derived from company and industry-specific sources
- The report covers pipeline products based on various stages of development ranging from pre-registration till discovery and undisclosed stages
- The report features descriptive drug profiles for the pipeline products which includes, product description, descriptive MoA, R&D brief, licensing and collaboration details & other developmental activities
- The report reviews key players involved in Interleukin 1 (IL1) targeted therapeutics and enlists all their major and minor projects
- The report assesses Interleukin 1 (IL1) targeted therapeutics based on mechanism of action (MoA), route of administration (RoA) and molecule type
- The report summarizes all the dormant and discontinued pipeline projects
- The report reviews latest news and deals related to Interleukin 1 (IL1) targeted therapeutics
Reasons to buy
- Gain strategically significant competitor information, analysis, and insights to formulate effective R&D strategies
- Identify emerging players with potentially strong product portfolio and create effective counter-strategies to gain competitive advantage
- Identify and understand the targeted therapy areas and indications for Interleukin 1 (IL1)
- Identify the use of drugs for target identification and drug repurposing
- Identify potential new clients or partners in the target demographic
- Develop strategic initiatives by understanding the focus areas of leading companies
- Plan mergers and acquisitions effectively by identifying key players and it's most promising pipeline therapeutics
- Devise corrective measures for pipeline projects by understanding Interleukin 1 (IL1) development landscape
- Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope
- Key Topics Covered:
- Introduction
- Report Coverage
- Interleukin 1 (IL1) - Overview
- Interleukin 1 (IL1) - Therapeutics Development
- Products under Development by Stage of Development
- Products under Development by Therapy Area
- Products under Development by Indication
- Products under Development by Companies
- Products under Development by Universities/Institutes
- Interleukin 1 (IL1) - Therapeutics Assessment
- Assessment by Mechanism of Action
- Assessment by Route of Administration
- Assessment by Molecule Type
- Interleukin 1 (IL1) - Companies Involved in Therapeutics Development
- 3SBio Inc
- Bioiberica SAU
- Devonian Health Group Inc
- Johnson & Johnson
- Komipharm International Co Ltd
- Link Health Group
- Mabpharm Ltd
- Moderna Therapeutics Inc
- Novartis AG
- Omnitura Therapeutics Inc
- Opsona Therapeutics Ltd
- Peptinov SAS
- R Pharm
- Regeneron Pharmaceuticals Inc
- Spherium Biomed SL
- Swedish Orphan Biovitrum AB
- Therapicon Srl
- TWi Biotechnology Inc
- XBiotech Inc
- Interleukin 1 (IL1) - Drug Profiles
- AC-203 - Drug Profile
- bermekimab - Drug Profile
- Interleukin 1 (IL1) - Dormant Products
- Interleukin 1 (IL1) - Discontinued Products
- Interleukin 1 (IL1) - Product Development Milestones
- Featured News & Press Releases
- Appendix
Companies Mentioned
- 3SBio Inc
- Bioiberica SAU
- Devonian Health Group Inc
- Johnson & Johnson
- Komipharm International Co Ltd
- Link Health Group
- Mabpharm Ltd
- Moderna Therapeutics Inc
- Novartis AG
- Omnitura Therapeutics Inc
- Opsona Therapeutics Ltd
- Peptinov SAS
- R Pharm
- Regeneron Pharmaceuticals Inc
- Spherium Biomed SL
- Swedish Orphan Biovitrum AB
- Therapicon Srl
- TWi Biotechnology Inc
- XBiotech Inc
For more information about this drug pipelines report visit https://www.researchandmarkets.com/r/tc5d7s
Contacts
ResearchAndMarkets.com
Laura Wood, Senior Press Manager
press@researchandmarkets.com
For E.S.T Office Hours Call 1-917-300-0470
For U.S./CAN Toll Free Call 1-800-526-8630
For GMT Office Hours Call +353-1-416-8900
게시글 찬성/반대
- 7추천
- 1반대
운영배심원의견
운영배심원의견이란
운영배심원(10인 이하)이 의견을 행사할 수 있습니다.
운영배심원 4인이 글 내리기에 의견을 행사하게 되면
해당 글의 추천수와 반대수를 비교하여 반대수가
추천수를 넘어서는 경우에는 해당 글이 블라인드 처리
됩니다.






댓글목록