Granulocyte-macrophage colony-stimulating factor (GM-CSF), which belongs to the colony-stimulating factor (CSF) superfamily and was originally identified as a hemopoietic growth factor, is mainly produced by lymphocytes and innate lymphoid cells. Nonhematopoietic cell populations such as fibroblasts, endothelial cells, and epithelial cells may also secrete GM-CSF in response to an activating stimulus. Currently, GM-CSF is considered to play a smaller role in homeostatic myelopoiesis in a steady state because GM-CSF-gene-deficient mice exhibit steady-state hematopoiesis and a virtually normal lifespan of granulocytes and monocytes [ 1]. During the course of inflammation, systemic GM-CSF dramatically increases monocyte and
neutrophil production from the bone marrow and plays an important role in the expansion and differentiation of myeloid cells [ 2]. Moreover, GM-CSF is deemed essential for resistance to local infection and normal pulmonary physiology. GM-CSF deficiency results in pulmonary alveolar proteinosis characterized by the failure to clear surfactant from the lungs due to a shortage of macrophages induced by GM-CSF.
GM-CSF is rarely detectable in the peripheral blood of healthy individuals and is expressed basally in nonsterile tissues such as the lung, gut, and skin. During inflammation, GM-CSF is principally produced by T helper (Th) cells and acts as a communication conduit between myeloid cells and tissue-invading lymphocytes. In an experimental autoimmune encephalomyelitis mouse model, the interleukin (IL)-7-STAT5 axis promotes the generation of GM-CSF/IL-3-producing Th cells, which are designated Th-GMs, exhibit a distinct transcriptional profile, and represent a novel subset of Th cells [ 3]. In another experimental autoimmune encephalomyelitis mouse model, it was reported that GM-CSF is critical for the proinflammatory functions of Th17 cells; GM-CSF blockade, but not IL-17 depletion, may efficiently inhibit tissue inflammation [ 4]. Therefore, GM-CSF is considered an important
proinflammatory cytokine and not only a supplementary medication for patients with neutropenia secondary to chemotherapy-induced myelosuppression.
Sepsis is a life-threatening medical condition caused by the entry of various microorganisms into the human bloodstream that triggers an uncontrolled inflammatory reaction [ 5]. The systemic inflammatory response syndrome/compensatory anti-inflammatory response state model or persistent inflammation-immunosuppression catabolism syndrome model are used to depict the immune status of sepsis patients. An increased number of peripheral GM-CSF-producing CD4+ T cells were shown to serve as a marker of severity in patients with sepsis [ 6]. However, exogenous GM-CSF administration improved the survival of patients with sepsis by enhancing phagocytosis by neutrophils and monocytes and increasing reactive oxygen species generation. Therefore, a better stratification of the immune status with a tailored approach is essential for the treatment of patients with sepsis. The expression of monocytic human leukocyte antigen DR (mHLA-DR) in fewer than 30% of cells, reduction in the
lipopolysaccharide (LPS)-induced TNF-α level to <200 pg/mL in whole blood, and an absolute lymphocyte count <1000 cells/mm3 are significant factors for immunoparalysis [ 7]. GM-CSF stimulates the production of neutrophils and monocytes by the bone marrow and maintains the activation and survival of monocytes. GM-CSF restored cytokine secretion in monocytes from sepsis patients by reducing apoptosis and increased CD71 and HLA-DR expression on monocytes upon synergistic coordination with LPS in vitro [ 8]. Many clinical studies have found that GM-CSF treatment has a potential benefit for patients with sepsis. In a prospective, randomized, open-label clinical study, GM-CSF treatment facilitated the recovery of the TNF-α response and prevented nosocomial infection in children with nonneutropenic, nontransplant, and severe multiple organ dysfunction syndrome [ 9]. Strong evidence has shown that mHLA-DR and plasma IL-6 levels are closely related to the outcome of recombinant human GM-CSF administration, and sepsis patients treated with GM-CSF have a shorter length of stay in the hospital or intensive care units [ 10]. In another randomized phase II trial, although GM-CSF-treated patients with sepsis showed improvement in PaO2/FiO2 and increased peripheral blood neutrophil counts, GM-CSF therapy did not reverse acute respiratory distress syndrome and had no effect on 30-day survival [ 11]. As GM-CSF application might lead to different results in severe sepsis at different stages, we suggest that biomarker-guided, individualized precision therapies based on the immune status may work in a well-defined patient population
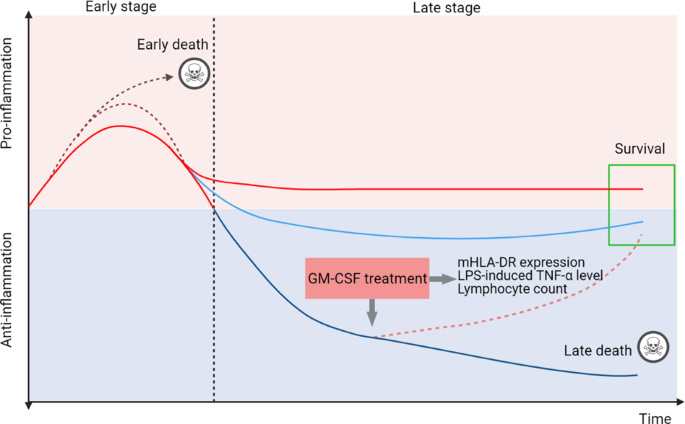
(Fig. 1).
Sepsis initiates a complex immune response with the concomitant occurrence of excessive systemic inflammation at an early stage and relative immunosuppression at the late stage. Some patients die in the early stage of the uncontrolled proinflammatory response, whereas some patients die during the late stage of irreversible immune suppression. Different markers are used to distinguish the immunological thresholds, including mHLA-DR expression in <30% of cells, LPS-induced TNF-α level <200 pg/mL in whole blood, and absolute lymphocyte counts <1000 cells/mm3. GM-CSF treatment is preferred for use in patients with severe immunosuppression. mHLA-DR monocytic human leukocyte antigen DR, LPS lipopolysaccharide.
Nature.com article: "Clinical trials have demonstrated the efficacy of GM-CSF blockade in the treatment of patients with COVID-19. GM-CSF neutralization with lenzilumab prevents immune hyperstimulation and is associated with faster clinical improvements and slower progression to severe disease or death in some high-risk COVID-19 patients with severe pneumonia [14]".
https://www.nature.com/articles/s41423-021-00719-3
Nature.com 기사: "임상시험은 COVID-19 환자 치료에 있어 GM-CSF 봉쇄의 효과를 보여주었다. 렌질루맵을 사용한 GM-CSF 중화는 면역 과잉자극을 예방하고 중증 질환이나 사망에 이르는 일부 고위험 환자에서 임상적 개선과 더 느린 진행과 관련이 있다.e 폐렴[14].
https://www.nature.com/articles/s41423-021-00719-3

 </picture>
</picture>




댓글목록